Since the isolation of the cDNA in 1994, the work on the mammalian MTHFR gene has resulted in significant advances in our understanding of its genomic organization, genetic variations and involvement in human disorders. Several important issues, however, remain to be addressed. Little is known about the regulation of this gene despite the fact that the enzyme links folate and homocysteine metabolism, and is involved in such critical cellular processes as DNA synthesis and DNA methylation.
Molecular Biology of Methylenetetrahydrofolate Reductase (MTHFR) and Overview of Mutations/Polymorphisms
Folate, B12 and MTHFR share a unique relationship.
While the first two are vitamins and the other one is an enzyme, they all play key roles in the metabolic processes of the body, especially in relation to homocysteine.
Folate, B12 And MTHFR
MTHFR stands for methylene-tetra-hydro-folate reductase, an enzyme that reduces 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate. Put simply, it converts vitamin B9 (folate) into its active form, L-methylfolate, also known as methylfolate.
Here’s a schematic of this conversion:
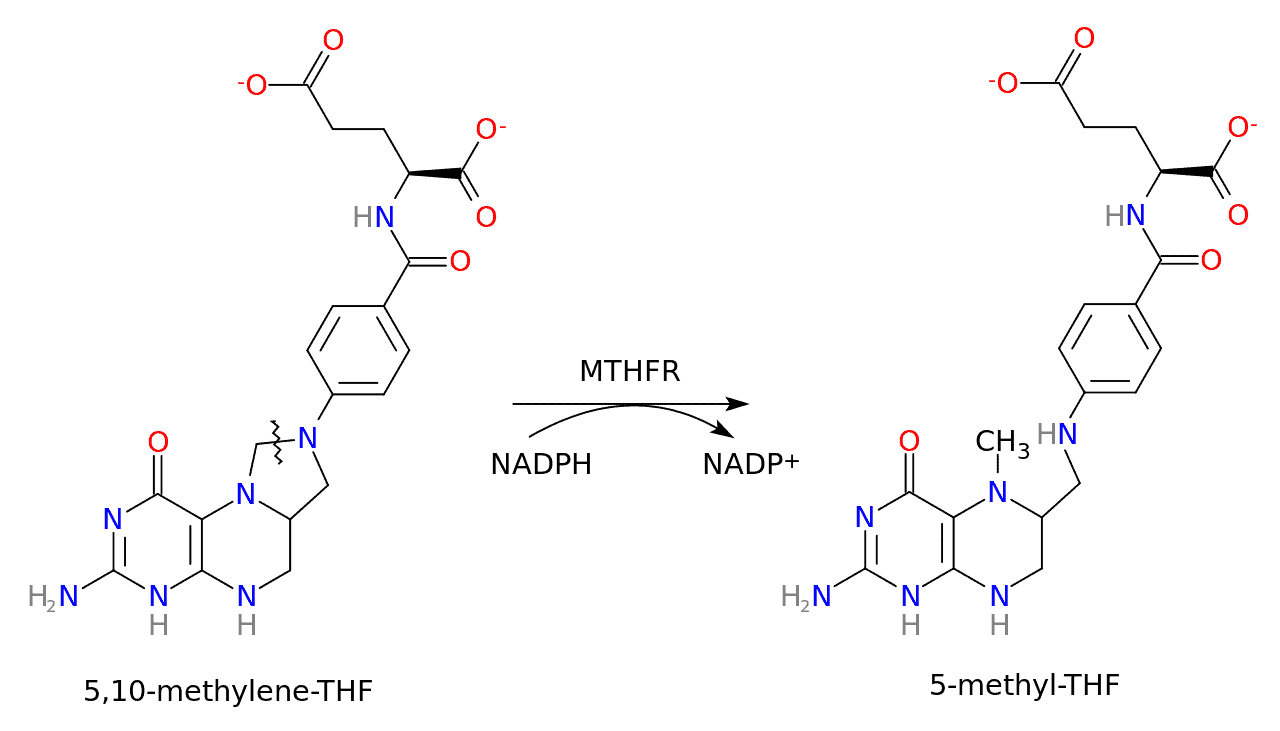
The MTHFR gene is located on the short arm of chromosome 1 (1p36.3) and encodes for dimeric proteins. It provides instructions for creating the MTHFR enzyme.
Again, the MTHFR enzyme provides us with methylfolate, which is a crucial methyl donor for the conversion of homocysteine to methionine. It does so by donating its methyl group (a carbon atom bonded to three hydrogen atoms) to a cobalamin (B12) molecule, forming methylcobalamin, which then donates it to homocysteine, forming methionine.
This methylation process of transferring the methyl group from one molecule to another plays a key role in DNA repair, neurotransmitter synthesis, hormone and gene regulation, immune function, cell division, and detoxification. Mutations in the MTHFR gene are very common, in particular C677T and A1298C. These two variants can reduce the activity of the enzyme, affecting the methylation cycle by producing less methylfolate.
In fact, MTHFR is the most asked-about gene by 23andMe customers.
However, not everyone with an MTHFR mutation will exhibit clinical symptoms or have significant methylation issues. The actual impact depends on whether the mutation is homozygous or heterozygous, and on other genetic and environmental factors.
For instance:
Women with two copies of C677T appear to have a slightly higher risk of having a child with a neural tube defect. However, according to the National Institute of Child Health and Human Development, folic acid supplementation reduces the risk of neural tube defects in all pregnant women, including women with an MTHFR variant.
When consuming the same amount of folic acid, people with the MTHFR 677 TT genotype (two copies of T, one copy from each parent) have on average 16% less folate in their blood than people with the MTHFR 677 CC genotype (two copies of C). In a meta-analysis of 40 studies, people with the 677 TT genotype had a strikingly similar 16% higher odds of coronary heart disease compared with those with the CC genotype:
These results support the hypothesis that impaired folate metabolism, resulting in high homocysteine levels, is causally related to increased risk of CHD.
MTHFR 677C→T Polymorphism and Risk of Coronary Heart Disease
A genotype is the combination of the genes (from both parents) responsible for a certain trait, in our case the function of the MTHFR enzyme.
One hypothesis is that C677T’s reduction in MTHFR activity is likely due to the fact that the alanine to valine amino acid change makes the enzyme more heat-sensitive.
The correlation between MTHFR-related elevated homocysteine and coronary heart disease is no way conclusive, as some evidence is conflicting. One notable example is this meta-analysis of MTHFR case-control studies, which raises a serious concern:
What if only Mendelian randomization studies that found an association have been published? Such publication bias would affect this aggregate result. Here, the researchers investigate the association of the MTHFR C677T polymorphism with CHD in unpublished datasets that have analyzed this polymorphism incidentally during other genetic studies.
The researchers obtained 19 unpublished datasets that contained data on the MTHFR C677T polymorphism in thousands of people with and without CHD. Meta-analysis of these datasets indicates that the excess CHD risk in TT homozygotes compared to CC homozygotes was 2% (much lower than predicted from the prospective observational studies), a nonsignificant difference (that is, it could have occurred by chance). When the probable folate status of the study populations (based on when national folic acid fortification legislation came into effect) was taken into account, there was still no evidence that TT homozygotes had an excess CHD risk. By contrast, in an updated meta-analysis of 86 published studies of the association of the polymorphism with CHD, the excess CHD risk in TT homozygotes compared to CC homozygotes was 15%. Finally, in a meta-analysis of randomized trials on the use of vitamin B supplements for homocysteine reduction, folate supplementation had no significant effect on the 5-year incidence of CHD.These analyses of unpublished datasets are consistent with lifelong moderate elevation of homocysteine levels having no significant effect on CHD risk. In other words, these findings indicate that circulating homocysteine levels within the normal range are not causally related to CHD risk. The meta-analysis of the randomized trials of folate supplementation also supports this conclusion. So why is there a discrepancy between these findings and those of meta-analyses of published Mendelian randomization studies? The discrepancy is too large to be dismissed as a chance finding, suggest the researchers, but could be the result of publication bias—some studies might have been prioritized for publication because of the positive nature of their results whereas the unpublished datasets used in this study would not have been affected by any failure to publish null results.
Overall, these findings reveal a serious example of publication bias and argue against the use of folate supplements as a means of reducing CHD risk.
Homocysteine and Coronary Heart Disease: Meta-analysis of MTHFR Case-Control Studies, Avoiding Publication Bias
Ultimately, the idea that a single, common genetic variant like C677T can cause so many unrelated health issues is absurd. The 1000 Genomes Project estimates that around 25% of the global population are C677T carriers. That’s without mentioning A1298C. It’s estimated that 40% of the general population have one of the two variants.
Gene variants are normal. With MTHFR, what started as mutations are now a common part of the human gene pool, just like blue eyes, lactose tolerance, and many others. Not all variants cause disease, especially if they are so incredibly common. While C677T and A1298C do decrease enzyme activity and make MTHFR function suboptimal, carriers can generally expect to have no trouble converting enough homocysteine to methionine.
Rarely, however, MTHFR activity may be so low that it can result in homocystinuria, leading to abnormal blood clotting, brittle bones, nearsightedness, and increased risk of having a child with a neural tube defect. However, it is extremely rare, with about one in a quarter million people being affected worldwide. It could also result from other genetic mutations, such as in the cystathionine-beta-synthase (CBS) gene.
What About Vitamin B12?
Methylcobalamin (methyl B12) is a cofactor for the enzyme methionine synthase, which converts homocysteine to methionine. But B12 takes the form of methylcobalamin only after it receives its methyl group from methylfolate, which again, MTHFR is responsible for producing. Still, B12 plays a pivotal role in reducing homocysteine levels. This is why the homocysteine test is a useful tool in helping diagnose B12 deficiencies.
On The Inter-Conversion of B12 Forms
The conversion of other B12 forms (cyanocobalamin, hydroxycobalamin, and adenosylcobalamin) into methylcobalamin is not dependent on MTHFR. Rather, other mechanisms facilitate these conversions. This ability to convert all forms of B12 to its active forms is usually very efficient.
In fact, when methylcobalamin donates its methyl group to homocysteine, the cobalamin (the B12) can then convert to hydroxocobalamin, a form of B12 that can act as a “scavenger” of nitric oxide radicals in the body. It’s also a precursor to both methylcobalamin and adenosylcobalamin.
It can be re-methylated to form methylcobalamin once again. When the body uses methylcobalamin, it typically does so for donating its methyl group. If more methylcobalamin is needed, the body can produce it through the process of remethylation, using methylfolate again as the methyl donor. This recycling is part of the body’s efficient use of B12, as it allows the same B12 molecule to participate in multiple rounds of methylation reactions.
This might seem like an inefficient way to do things, using methylcobalamin just to donate its methyl group and then having to remethylate it. However, this dynamic and cyclical process allows the body to maintain a balance of various forms of B12 and ensure that the right form of the vitamin is available for the specific reactions required at any given time.
This mechanism is regulated by cellular requirements. If the body needs more methylcobalamin, then the B12 molecule can be remethylated. This interplay stresses the importance of both B12 and folate in the methylation cycle.
So, why take methylcobalamin if the body can convert other forms to it even in the presence of MTHFR mutations? Because you can. Of all the B12 forms, it is the only one that comes with a methyl group, of which many people don’t have enough. Think of it as both a source of B12 and methyl groups.
Keep in mind, while the logic of the methylation cycle and MTHFR variants suggests a preference for methylcobalamin, do consider other factors. For instance, some people might have issues related to the cellular uptake of B12, which may suggest supplementing with adenosylcobalamin too.
MTHFR and B12
The MTHFR enzyme specifically pertains to folate metabolism. And while the MTHFR variants primarily impact folate metabolism, the interconnectedness of the methylation cycle means that it indirectly affects vitamin B12 utilization as well.
If someone has a less efficient MTHFR variant, there would be less methylfolate, and therefore less methylcobalamin for converting homocysteine. If there’s also a B12 deficiency in the mix, then the risk of high homocysteine levels is amplified.
So, again, the MTHFR gene instructs the MTHFR enzyme how to convert folate into its active form. This active form of folate then donates its methyl group to a B12 molecule, creating methylcobalamin. Finally, the enzyme methionine synthase uses this methylcobalamin to convert homocysteine into methionine.
This, in short, is the relationship between folate, B12 and MTHFR.
Best Forms of Folate & B12 For MTHFR
If you carry the C677T or A1298C variant, there’s little reason not to choose methylated forms of B12 and folate as your forms of choice. These are, of course, methylcobalamin and methylfolate. By supplementing with these two active forms, you’ll support the methylation cycle and assist in homocysteine metabolism.
In the context of MTHFR variants, providing the body with methylfolate will help counter its own, reduced production of methylfolate. Also, methyl B12 will directly give your body what it needs for converting homocysteine to methionine. We suggest supplementing with both, since doing so is cheap and easy, and will ensure that the components of the homocysteine-to-methionine conversion pathway are readily available.
Also, just like methylcobalamin, methylfolate carries a methyl group, further helping replenishing your methyl group stores, regardless of the folate.
But, is methylfolate as effective as folic acid?
Folic acid is a cheap, synthetic form of vitamin B9, which you’ll find in fortified foods and many dietary supplements. It’s not the form of folate naturally present in food. On the other hand, methylfolate is the biologically-active form of B9 and what the body uses directly. It essentially comes ready-to-use unlike folic acid, where the body must undergo a series of enzymatic conversions to transform it into the active form.
As for effectiveness, here’s a study in which healthy women were randomly assigned to consume 400 µg of folic acid, 416 µg of methylfolate (the bioequivalent dose of folic acid), and 208 µg of methylfolate (half dose). Each group saw increases in plasma folate and decreases in homocysteine, suggesting that methylfolate behaves predictably.
The authors concluded that due to the high prevalence of MTHFR variants and how crucial it is to assure pregnant women get adequate supplementation, “L-methylfolate may be the best option to avoid blood folate deficiencies.” The study also notes that excessive ingestion of folic acid could mask an undiagnosed B12 deficiency, and that it was unlikely that methylfolate would mask a B12 deficiency in the same way.
For all these reasons, methylfolate is the better option.
The natural form of folate, 5-methylTHF (methylfolate), offers several advantages compared to folic acid: it does not mask B12 deficiency, it is already a biologically active form, it does not cause unmetabolized folic acid in blood, and it is absorbed and utilized at least as well as folic acid.
Is 5-methyltetrahydrofolate an alternative to folic acid for the prevention of neural tube defects?
Testing for MTHFR Variants
To know if you carry a C677T or A1298C variant in the MTHFR gene, you can either try to order genetic testing for MTHFR mutations through your healthcare provider, or simply get an MTHFR testing home kit, which may be both quicker and cheaper.
However, we honestly don’t think it’s necessary, unless you’re curious.
Again, up to 50% of people carry at least a single copy of one of the variants. Carrying it doesn’t automatically mean you’re not converting enough homocysteine to methionine, or that you necessarily have to supplement. These MTHFR gene variants are so common, people should not interpret their genotypes as having an effect on their health.
Unless, of course, the clinical picture says otherwise.
Currently, the American College of Obstetricians and Gynecologists does not recommend testing for MTHFR variants. Why pay for an expensive genetic test when you can measure homocysteine directly with a simple blood test? If blood numbers are high, treatment is simple. Supplementing with folate will bypass the need for the MTHFR enzyme.
If you think you may also be suffering from a vitamin B12 deficiency, supplement with methylcobalamin as well. A good rule of thumb is to supplement if your B12 levels are lower than 500 (read this article to understand why). In this case, you can go for one supplement combining both methylcobalamin and methylfolate.
Summary
Despite lots of research – and lots of buzz – the existing scientific data doesn’t support the vast majority of claims that common MTHFR variants impact human health. Some websites have spread the idea that having one or two copies of an MTHFR variant can lead to dozens of negative health consequences. There are a couple problems with this claim. First, it’s unlikely that variants in a single gene could cause dozens of unrelated health problems. Second, the C677T and A1298C variants are very common: in some ethnicities, more than 50 percent of people have at least one copy of one of these variants. Most disease-causing genetic variants are not this common.
23andMe
To sum up, the MTHFR enzyme, as well as folate and B12, all play vital roles in the body’s metabolic processes, especially in the metabolism of homocysteine.
The MTHFR gene contains instructions for making the enzyme, which is responsible for converting folate into its active form, methylfolate. This form further donates its methyl group to a B12 molecule, forming methylcobalamin. The enzyme methionine synthase then uses methylcobalamin to convert homocysteine into methionine.
Two common variants in the MTHFR gene are C677T and A1298C. When the gene has either of these two variants, the resulting MTHFR enzyme is slightly less active, which can lead to lower levels of folate, and higher levels of homocysteine.
However, note that individual needs can vary, and an MTHFR variant doesn’t necessarily mean you need supplementation or will benefit from it in the same way.
If you choose to supplement, we suggest both methyl-B12 and methyl-folate. The two are ready-to-use and support the methylation cycle and homocysteine reduction. They also come pre-loaded with a methyl group, which is useful. If you can’t absorb B12 through the stomach, you can order injectable methylcobalamin instead.
For people with MTHFR mutations, supplementation with methylfolate will be more beneficial than folic acid, as they might have a reduced capacity to turn folic acid into methylfolate. Also, excessive intake of folic acid can lead to the unmetabolized folic acid accumulating in the bloodstream, a topic which has raised concerns.
Best of luck.