Updated on
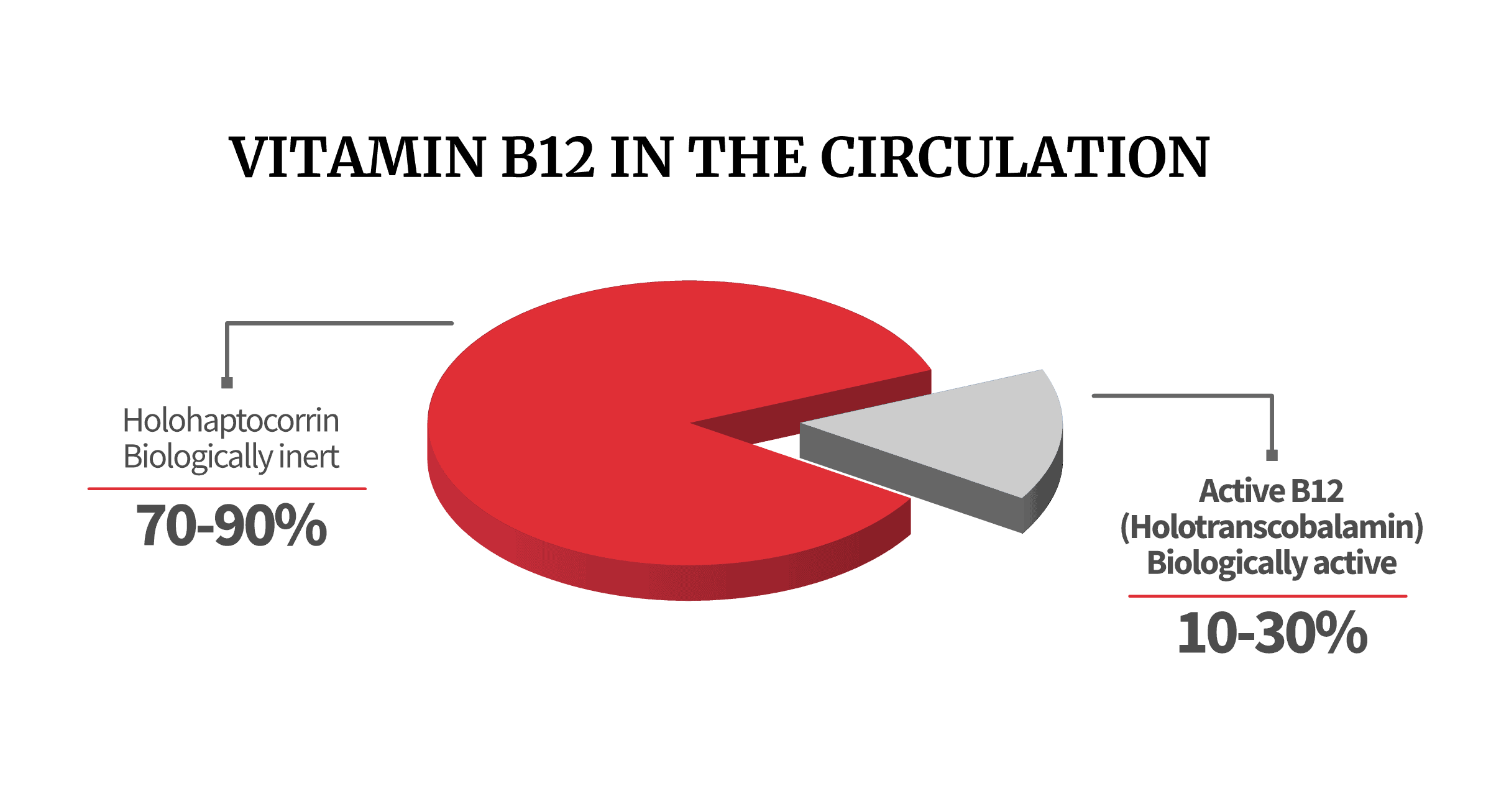
Holotranscobalamin represents only 10-30% of the B12 circulating in the blood but is the only form of B12 that is taken up and used by cells of the body, hence its other name – active B12.
Active-B12, the next level of B12 testing; Axis-Shield
The active-B12 test (or holoTC / holotranscobalamin test) from Axis-Shield is a novel method for assessing vitamin B12 status. Axis-Shield claims the test offers improved accuracy, sensitivity, and specificity compared to the regular total-B12 blood test.
Let’s explore it in detail.
What Is Active B12 (HoloTC)?
Approximately one-quarter of circulating vitamin B-12 binds to transcobalamin and is thereby available for the cells of the body. For this reason, holoTC is also referred to as active vitamin B-12.
Holotranscobalamin, a marker of vitamin B-12 status
The selective uptake of cobalamin by humans requires three transporting proteins: intrinsic factor (IF), transcobalamin (TC), and haptocorrin (HC). IF is responsible for intestinal uptake of vitamin B12. TC is the major plasma transporter. HC seems to be a scavenging protein.
Mechanisms of Discrimination between Cobalamins and Their Natural Analogues during Their Binding to the Specific B12-Transporting Proteins
There are three carrier proteins in the body that transport B12. The most well known is intrinsic factor (IF), against which the body creates antibodies in cases of pernicious anemia, while the other two are transcobalamin (TC) and haptocorrin (HC).
When transcobalamin and haptocorrin bind to the B12, the resulting complexes are known as holotranscobalamin (holoTC) and holohaptocorrin (holoHC), respectively.
Of the three carrier proteins, only transcobalamin can carry the B12 that is absorbed into the ileum, and move it further down to the tissues. Once there, the holoTC complex (that is, the “active B12”) is integrated through a specific receptor-mediated uptake, delivering B12 into the cells, where it acts as a co-enzyme for vital cellular functions.
What happens to the remaining B12 circulating in the blood? It has bound to haptocorrin, creating the biologically-inert complex holoHC. We’re not fans of the regular B12 blood test, because it fails to distinguish between holoTC and holoHC. This is a serious concern, because holoTC can make up as little as 10% of your total B12.
As a result, a real B12 deficiency can easily exist within what looks like a normal total-B12 status, and many genuine B12 deficiency cases end up undiagnosed.
Testing For Active B12 vs Total B12
From these results it can be concluded that serum concentration of holoTC is a much better predictor of B12 status than total B12.
The usefulness of holotranscobalamin in predicting vitamin B12 status in different clinical settings
HoloTC (“active B12”) is the only B12 complex available for cell and tissue absorption. However, since it may be as low as 10% of your total B12 levels, a regular B12 blood test often gives a misleading picture. Consider how vital holoTC is compared to holoHC:
There is a genetic condition in which the body lacks haptocorrin. However, it’s not life-threatening, and doctors often discover it accidentally. In contrast, the absence of transcobalamin is very serious, and it manifests as the typical symptoms of deficiency in B12. Doctors often diagnose it shortly after birth due to failure to thrive, and it requires immediate B12 therapy, otherwise permanent neurological damage takes place.
In such cases, and in other situations where transcobalamin is compromised, a normal B12 blood test may show false healthy B12 levels, whereas an active-B12 test could have discovered a deficiency. Thus, some predict that the active-B12 blood test will one day replace the normal test as the default diagnostic tool. There are good reasons:
Compared to total cobalamin, we observed a better performance of holoTC assay in detecting elevated concentrations of MMA in subjects with normal renal function. The majority of subjects with combined low holoTC and elevated MMA had normal concentrations of total cobalamin. HoloTC can be used as a first line parameter in detecting cobalamin deficiency.
Holotranscobalamin in laboratory diagnosis of cobalamin deficiency compared to total cobalamin and methylmalonic acid
This case study demonstrated that 5 different Total-B12 assays from 4 manufacturers all gave falsely elevated results in a patient with confirmed pernicious anaemia. The same failing described by Carmel, Agrawal, Yang and Cook is again apparent in this case study and demonstrates why Total-B12 results can be unreliable.
FALSELY ELEVATED COBALAMIN CONCENTRATION IN MULTIPLE ASSAYS IN A PATIENT WITH PERNICIOUS ANAEMIA: A CASE STUDY
After excluding 20 cases of incident dementia, increased tHcy remained associated with poorer performance in episodic memory, execution functions and verbal expression. Higher holoTC levels tended to be related to better performance in executive functions and psychomotor speed, while elevated serum folate concentrations were significantly related to higher scores in global cognition and verbal expression tests.
Serum homocysteine, holotranscobalamin, folate and cognition in the elderly
We confirm a decrease in cobalamins during pregnancy, and report that the active part of cobalamins (holotranscobalamin, holoTC) remains unchanged. The decrease in cobalamins is explained by a decreased holohaptocorrin (holoHC), suggesting that holoTC rather than cobalamins should be used as a marker of vitamin B12 deficiency during pregnancy.
Holotranscobalamin remains unchanged during pregnancy
No association between serum vitamin B₁₂ concentrations and cognitive decline or dementia was found. However, four studies that used newer biomarkers of vitamin B₁₂ status (methylmalonic acid and holotranscobalamin (holoTC)) showed associations between poor vitamin B₁₂ status and the increased risk of cognitive decline or dementia diagnosis.
Vitamin B12 status, cognitive decline and dementia
HoloTC has a better diagnostic accuracy than vitamin B(12) and can replace the existing vitamin B(12) assay as a primary screening test in patients suspected of vitamin B(12) deficiency.
Screening for metabolic vitamin B12 deficiency by holotranscobalamin in patients suspected of vitamin B12 deficiency: a multicentre study
The active-B12 test uses a novel monoclonal antibody that cuts out the need for complex preliminary steps, thus eliminating a potential source of inconsistency. Another benefit is that holoTC has a shorter circulating half-life compared to holoHC. So, the idea is that as you’re approaching a negative B12 balance, the earliest change that actually occurs is likely to be a decrease in the levels of active B12 specifically.
All that being said…
The Name “Active-B12 Test” Is Misleading
Our primary issue with the active-B12 test is the misleading choice of name. Nobody should have named it that way – it’s simply a holoTC test. Why does it matter?
Because the active-B12 test also tests for inactive B12.
B12 comes in many forms, depending on the ligand on its cobalt atom. While TC does bind to the active forms adenosylcobalamin and methylcobalamin, it can also bind to co(II)cobalamin, aquocobalamin, cyanocobalamin, nitrosylcobalamin, hydroxocobalamin, cysteinylcobalamin, aquocobalamin, glutathionylcobalamin, thiocyantocobalamin, sulfitocobalamin, or chlorocobalamin, none of which are biologically active.
TC is more promiscuous than IF, although not as much as HC:
Three proteins, intrinsic factor (IF), transcobalamin (TC), and haptocorrin (HC), all have an extremely high affinity for the cobalamins but discriminate these physiological ligands from Cbl analogues with different efficiencies decreasing in the following order: IF > TC > HC.
Mechanisms of Discrimination between Cobalamins and Their Natural Analogues during Their Binding to the Specific B12-Transporting Proteins
As you can see, TC can still bind to analogues, which are inactive compounds structurally similar to B12. In a lab, you can even bind nanoparticles or polymers to the cobalt atom, and TC will bind to them. The structural change that TC undergoes when it becomes holoTC is what allows the monoclonal antibody in the test to recognize it as such. However, it has nothing to do with whether the B12 is active or not.
Put differently, the active-B12 test measures the absolute amount of any B12 form bound to transcobalamin. The test is quantitative, not qualitative, since it can’t distinguish analogues from methylcobalamin and adenosylcobalamin, the true active forms and cofactors for the enzymes methionine synthase and MMA CoA mutase.
However, the above makes sense in-vitro. In the human body, things get a little more complicated. Take a look at the diagram we made for vitamin B12 absorption:
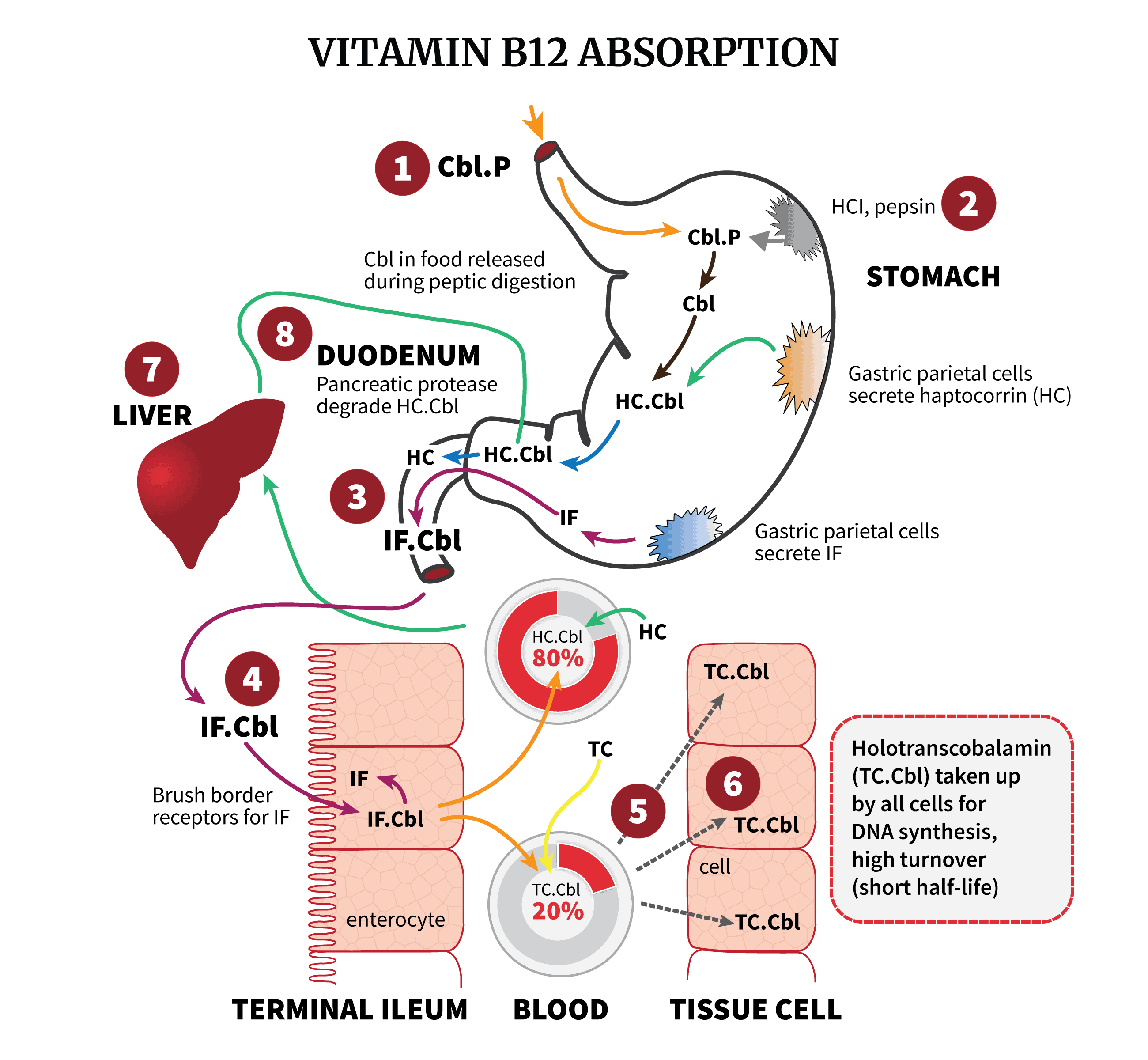
As shown, IF, which as the highest specificity against analogues, filters cobalamins at the intestine. At step 5 in the blood, HC, the least selective, captures spillover, sequestering the remaining analogues into what seems to be a holding pattern for long-term storage. TC then carries the “clean” B12, around 20% of blood cobalamins. The crucial point:
Transcobalamin inherits the selectivity of intrinsic factor – because IF filters at the intestine, before TC gets the chance to bind to anything.
Notice the elegant system:
1. IF filters at the intestine. Its high discrimination prevents most dietary analogues from entering the terminal ileum and becoming IF-B12 complexes.
2. HC scavenges remaining analogues. It sponges up most dietary analogues, even those that escape IF and could otherwise bind to TC. The protective role of HC appears to be exactly this sequestering of analogues before they can saturate IF or TC. HC can deplete intestinal IF of analogues like cobinamide and pseudo-B12 within minutes.
3. TC binds to B12. By the time B12 has reached the blood, most analogues had been sequestered by HC. HoloTC should bind to true (active and inactive) cobalamins.
In short, the body maximizes the chance TC encounters true B12 through the upstream IF and HC filtration system. It’s an elegant protective hierarchy rather than TC’s own selectivity. However, inactive forms of B12 can still pass through. For instance, IF and TC picked up inactive cyano B12 with identical affinity as they did to active adenosyl B12. If it wants to be absorbed, cyano B12 must then convert to an active form.
And so, it is a little complex. Because even though the test does include inactive forms bound to transcobalamin, these complexes at least have a chance to convert:
After proteolysis of HC in the duodenum, Cbl is passed on to IF. Mucosal cells in the ileum absorb the IF–Cbl complex via endocytosis mediated by a specific receptor. In the enterocyte, the IF–Cbl complex is degraded and Cbl is transferred to TC which delivers Cbl to cells via the blood. Only the fraction of Cbl bound to TC is taken up via endocytosis by a specific receptor on most cell types. HC that is also present in plasma cannot facilitate cellular uptake of Cbl except in hepatocytes. TC–Cbl is degraded in lysosomes to release Cbl for further conversion into the important cofactors.
Structural study on ligand specificity of human vitamin B12 transporters
However, at the time of testing, the inactive forms inside the holoTC complexes are still inactive. As a result, we think calling it a holoTC test is more appropriate.
Any Expert Consensus?
Experts agreed that active-B12 offers a more reliable assessment of vitamin B12 status and as an output from the discussion a consensus statement was produced: “Emerging evidence indicates that active-B12 is a more reliable marker of B12 status than serum vitamin B12”.
Active-B12, the next level of B12 testing; Axis-Shield
In March 2012, a panel of nine recognized experts (Drs. Wolfgang Herrmann, Rima Obeid, Ralph Green, Donald Jacobsen, Dominic Harrington, Per Magne Ueland, Jan Lindemans, Ebba Nexø, and Anne Molloy) issued a consensus statement on the improved utility of the active-B12 assay over total B12. They have suggested the holotranscobalamin test be treated as the optimal marker for true B12 status. Others are in line:
Active-B12 has fulfilled our expectations in being a robust assay that improves the assessment of vitamin B12 status. It provides reassurance to us that we have contributed to improved patient care. The routine availability of this assay differentiates our laboratory as a progressive provider of quality pathology.
Dr .Ken Sikaris, Melbourne Pathology, Australia
Serum holoTC was the best predictor, with area under the ROC curve (95% CI) 0.90 (0.86-0.93), and this was significantly better than the next best predictors; serum cobalamin, 0.80 (0.75-0.85), and MMA, 0.78 (0.72-0.83). This study supports the use of holoTC as the first-line diagnostic procedure for vitamin B₁₂ status.
Diagnostic Accuracy of Holotranscobalamin, Methylmalonic Acid, Serum Cobalamin, and Other Indicators of Tissue Vitamin B12 Status in the Elderly
HoloTC levels were more sensitive than the serum vitamin B12 levels for indicating vitamin B12 status. If the serum vitamin B12 level is 151-300 pmol/L, the levels of holoTC alone or in combination with serum vitamin B12 levels are likely to be more useful markers than serum vitamin B12 levels alone.
Relationship between the Levels of Holotranscobalamin and Vitamin B12
Testing for vitamin B12 deficiency should start with holotranscobalamin measurement. Holotranscobalamin between 23 and 75 pM should be followed by MMA testing that can filter substantial number of deficient cases in the grey range in individuals with normal renal function. This diagnostic strategy may significantly improve assessing vitamin B12 deficiency.
UTILITY AND LIMITATIONS OF BIOCHEMICAL MARKERS OF VITAMIN B12 DEFICIENCY
Total serum cobalamin is neither sensitive nor it is specific for cobalamin deficiency. This might explain why many deficient subjects would be overlooked by utilizing total cobalamin as status marker. Concentration of holoTC in serum is an earlier marker that becomes decreased before total serum cobalamin.
Cobalamin deficiency
However, there is no consensus between all experts. In addition, the latest Medical Policy review considers diagnosing B12 deficiency with a holoTC test investigational:
There is not enough research to know if or how well serum holoTC testing works as an alternative to total serum cobalamin, levels of methylmalonic acid (MMA), or homocysteine to improve diagnosis and management of vitamin B12 deficiency. No clinical guidelines based on research recommend holoTC testing. Therefore, holoTC testing is considered investigational for all indications.
Others completely oppose the test. We recently talked to Dr. Gregory Russell-Jones (PhD):
I have been working with vitamin B12, vitamin B12 analogues and the use of vitamin B12 as a targeting agent for some 40 years now. Part of that work involved cloning of Intrinsic Factor (IF), determining the active site of IF for vitamin B12 using photoactivatable cross-linkers, and developing VB12-linked targeting agents for cancer chemotherapeutics.
More recently I have been looking at serum vitamin B12 levels and correlating them with functional vitamin B12 deficiency. This has also involved the use of many analogues of vitamin B12. In addition I have used experiments with radioactive derivatives to look at binding to and displacement of vitamin B12 analogues from intrinsic factor, transcobalamin and haptocorrin. I have also defined a condition, known now in the literature as Paradoxical B12 Deficiency. In this deficiency vitamin B12 levels in serum can be normal (well, according to the pathology labs definition of normal which varies from country to country and lab to lab, so it is nowhere near standard), or elevated.
Through these studies I have come to realize that the only true measurement of vitamin B12 activity is to look at functional markers of deficiency of which there are many, including the standard MMA and homocysteine, but there are plenty more including HVA/VMA/QA/5HIAA, pyroglutamic acid, HMG, citrate, SAM:SAH, GSH:GSSG, creatinine and choline, to name a few. These studies have also shown that the so-called active B12 test is a complete and utter waste of time and money and should NEVER have been approved for use.The fact that transcobalamin can bind to any corrinoid does not mean the corrinoid is active. In fact in many instances, and particularly in cases when circulating B12 is high, the majority of the B12 is Co(II)B12. This is such basic chemistry. The best tests are either MMA, Hcy or the Organic Acids Test where you can measure functional B12 sufficiency.
The National Health and Nutrition Survey (NHANES) offers using a distinctive dual-marker approach, using one B12 concentration biomarker, total serum B12 or holoTC, together with one functional biomarker like MMA or homocysteine:
Given each biomarker’s sensitivity and specificity problems, the results of a single biomarker measurement would be difficult to interpret. But if NHANES can measure only one biomarker, some experts felt that this measure should reflect the broad range of vitamin B-12 status in the US population. Vitamin B-12 and holoTC increase continuously with increasing intakes, at least until vitamin B-12 saturates the transport proteins, and therefore reflect a broad range of intakes and status. Other roundtable experts felt that the sensitivity of MMA and tHcy to early inadequacies in vitamin B-12 status and marginal vitamin B-12 intakes could support the use of either one as the sole NHANES biomarker.
However, the roundtable strongly urged that, given the sensitivity and specificity problems of all vitamin B-12–related biomarkers, future NHANES should concurrently measure at least one biomarker of circulating concentrations of vitamin B-12 (vitamin B-12 or holoTC) and one biomarker of functional vitamin B-12 status (MMA or tHcy). If a choice must be made between serum vitamin B-12 and holoTC, holoTC has the advantage of not being affected by “false-positive” low vitamin B-12 concentrations such as those caused by genetically determined haptocorrin deficiencies. These conditions result in low circulating vitamin B-12 concentrations but have no adverse effect on vitamin B-12 status because they do not affect holoTC, which is the universal transport protein for vitamin B-12 uptake in all cells. However, the roundtable also recognized that holoTC measurement is new and would benefit from additional performance studies to engender full confidence in the use of holoTC for population-based surveys. Therefore, at this time, the roundtable agreed that serum vitamin B-12 is preferable over holoTC as the measure of circulating concentrations of vitamin B-12. MMA is preferable to tHcy if a choice must be made between these biomarkers because it increases with vitamin B-12 inadequacy, but not with folate inadequacy, whereas both of these nutrient deficiencies affect tHcy.
Biomarkers of vitamin B-12 status in NHANES: a roundtable summary
Some other research shows that the active-B12 test does have a higher diagnostic accuracy than the total-B12 test. Using MMA (a functional marker) as gold standard, HoloTC correctly identified 98.9% of the B12 deficiency cases detected by high MMA levels, while the total-B12 test only identified 63%. That being said, in half the cases where MMA levels were normal, both holoTC and total-B12 gave false positives.
In other words, low holoTC can classify someone as deficient even though their MMA levels – considered a definitive metabolic marker for B12 deficiency – are normal. So while holoTC is highly sensitive and appears good for ruling in a deficiency, it is less reliable for ruling out a deficiency on its own. NHANES’s idea to use a combination of markers (holoTC or total B12 + MMA or homocysteine) is good thinking.
Urinary MMA is another interesting test that is more available now than in the past, where it was only obtainable through Dr. Norman’s website. It is useful in older patients with impaired renal function, because the latter affects uMMA less than serum MMA:
We prospectively studied 127 patients with a plasma B12 between 201 and 350 ng/L. We noticed that uMMA/C was not dependent on renal function, contrary to plasma homocysteine and plasma methylmalonic acid. uMMA/C showed a perspective diagnostic performance and the threshold of 1.45 umol/mmol presented a high degree of specificity. In conclusion, uMMA/C is a promising biomarker to assess vitamin B12 status in doubtful cases, notably during renal impairment.
Diagnostic Performances of Urinary Methylmalonic Acid/Creatinine Ratio in Vitamin B12 Deficiency
Another interesting paper discusses the disagreement:
Reviews, presentations, commentary and letters, on the use of HoloTC as an earlier and more sensitive indicator of vitamin B12 deficiency than total vitamin B12, have been mixed. Several authors supported the claim that HoloTC was superior. Others raised concerns about the clinical utility of the test.
Questioning the superiority of the HoloTC test, Carmel stated that “what low holo-TC concentrations really tell us remains elusive.”
There are four problematic aspects of HoloTC, where there are major differences between authors: the value of the half-life of HoloTC; the correlation between HoloTC and total vitamin B12; sensitivity of HoloTC to recent absorption of vitamin B12; the value of HoloTC cut-off to detect negative vitamin B12 balance. The latter two differences are the most important because they raise the questions of what it is that HoloTC concentration actually indicates, and whether or not it is possible to use it to reliably detect the early onset of B12 deficiency.
As noted by Golding, it is possible to alter the apparent relative sensitivity of any pair of analytes by selectively changing the cut-off value of one or both of them. As demonstrated in that experiment, selecting different cut-off values for total vitamin B12 changed the relative sensitivities of HoloTC and total vitamin B12; the same applies to the cut-off value for HoloTC. When the HoloTC cut-off is increased, the sensitivity to vitamin B12 deficiency is increased, but the specificity of the test is reduced, increasing the number of false positive results. Conversely, if the HoloTC cut-off is decreased, the specificity of the test is increased, but the sensitivity of the test is reduced, increasing the number of false negative results.From their ROC charts, Heil selected MMA > 0.45 µmol/L to define vitamin B12 deficiency. With a HoloTC cut-off value of <21 pmol/L, the specificity was impressively high at 88 % but the sensitivity was only 64 %. By selecting the best trade-off between sensitivity and specificity for HoloTC, with a HoloTC cut-off value of <32 pmol/L, they obtained an impressive HoloTC sensitivity of 83 % but a specificity of only 60 %. To overcome this problem, as did Herrmann and Obeid, the authors suggest using MMA as a secondary test to confirm vitamin B12 deficiency in cases where HoloTC is <32 pmol/L. This approach would have failed to detect any vitamin B12 deficiency in the subject of Golding’s experiment because, despite overt metabolic disturbance evident from raised MMA concentration, and the onset of severe symptoms including peripheral neuropathy, HoloTC concentration never fell below 33 pmol/L.
Holotranscobalamin (HoloTC, Active-B12) and Herbert’s model for the development of vitamin B12deficiency: a review and alternative hypothesis
Anyhow, feel free to go through the data and make up your judgment.
Victor Herbert & Holotranscobalamin
We can’t discuss active B12 without mentioning Dr. Victor Herbert, a physician and hematologist who was the prime proponent for testing for holoTC, or “active-B12” levels.
Dr. Herbert was considered an expert on the metabolism of B12 and folate, on which he had authored several scientific papers, some of which are considered ground-breaking, landmark classics.
During his career, he was Professor of Medicine at the Mount Sinai School of Medicine in New York and Chief of Mount Sinai’s Hematology and Nutrition Research Laboratory in the Bronx Veterans Affairs Medical Center.

Dr. Herbert was perhaps most famous for his self-experiment, where he induced folate deficiency in himself by eating a diet of boiled vegetables. He almost killed himself in the process, waking up unable to walk. He did all that, in order to document the stages and progress of folate deficiency up until its end point of megaloblastic anaemia.
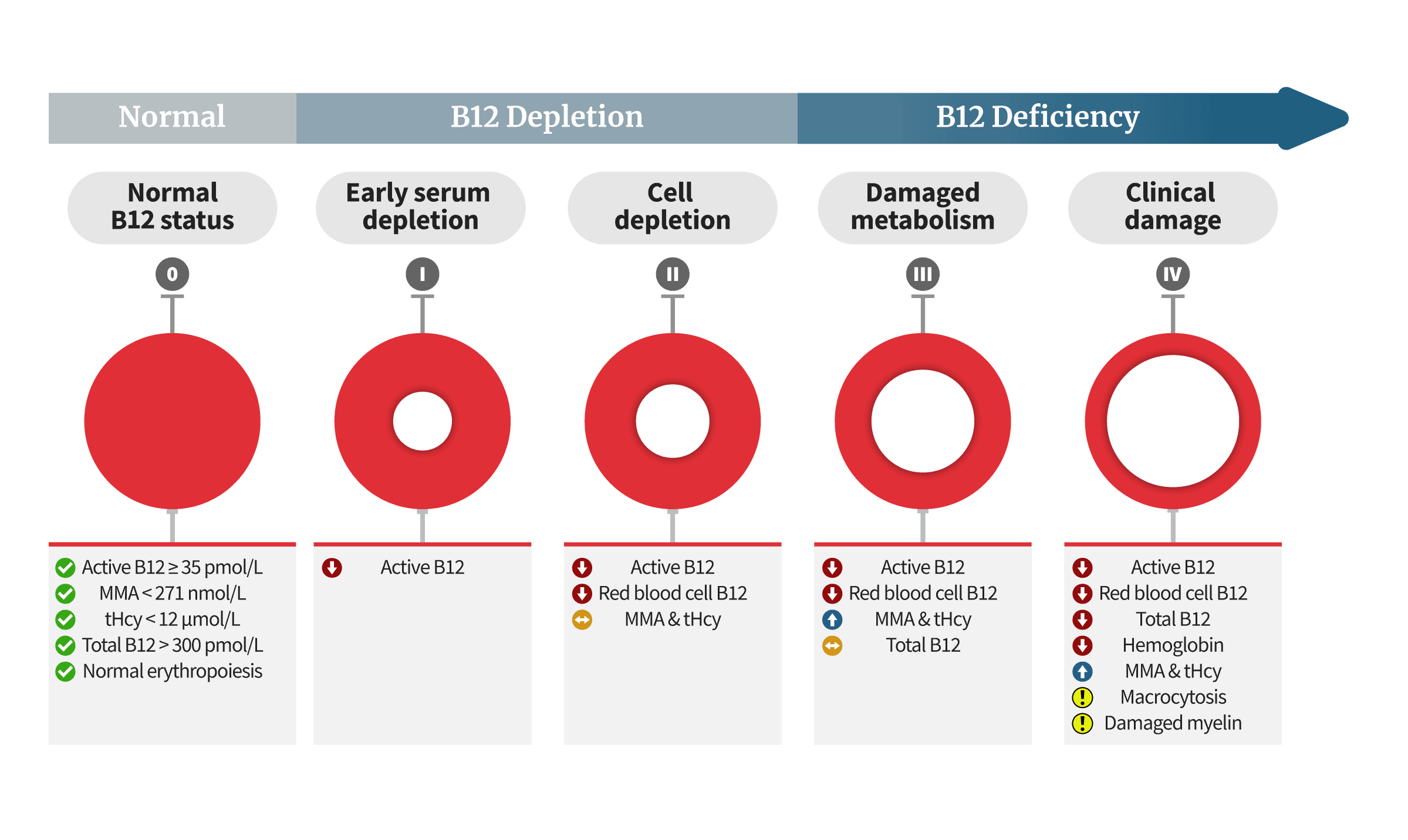
Below is a diagram showing an adaptation of Dr. Herbert’s proposed model. It describes the biochemical, metabolic, and clinical changes that take place as vitamin B12 repletion progresses to depletion, and finally to clinical B12 deficiency:
About 40 years after his experiment, on November 19, 2002, Dr. Herbert died at his home in NY. Weeks later, at a forum held in Philadelphia, Dr. Ralph Green read a tribute:
On Christmas Day 1961, he reached the end of one such study. Unable to move from severe weakness in both lower limbs induced by potassium deficiency caused by ingestion of an experimental diet, and having lost 26 pounds, Dr. Herbert terminated what came to be regarded as a classic self experiment. He had induced in himself nutritional folate deficiency by eating a diet of foods re-cooked to destroy all traces of folic acid. He had, as a consequence, developed megaloblastic anemia. Through this experiment, Dr. Herbert conclusively proved the tie between folate deficiency and megaloblastic anemia. He was also able to carefully document the sequence of changes in the development of folate deficiency. It was this spirit of scientific curiosity and indomitable grit and determination that characterized Victor Herbert’s life and career.
Obituary, Victor Herbert, M.D., J.D.
If you’re interested, here’s another article in memory of Victor Herbert, written by Dr. Charles Halstedand, and published in the American Journal of Clinical Nutrition. For his contribution to the field of vitamin B12 and to our understanding of its metabolism, we would like to thank Dr. Herbert from the bottom of our heart.
According to Herbert’s model, the first step of B12 deficiency is lower active-B12 levels. This is a core argument in favor of the active-B12 blood test becoming the first line of defense in diagnosing B12 deficiency. If Herbert’s model is accurate, it may help catch cases while they’re still recoverable and easy to treat. However, some doubt it:
The problems with Herbert’s model
The absence of an agreed single value for the HoloTC cut-off, together with uncertainty about whether HoloTC represents recent absorption of vitamin B12 or long-term status or both, must raise questions about Herbert’s hypothesis. Some of the most recent original research challenges Herbert’s model for the “Sequential stages in the development of vitamin B12 deficiency”. In reporting their original research results, Remacha commented that “These data do not support holoTC as the earliest marker of Cbl deficiency and challenge the classification in stages of Cbl deficiency”.In Herbert’s revised 1994 model, the change from Normal to Early Negative Vitamin B 12 Balance is marked by a fall in HoloTC concentration from >37 to <30 pmol/L. Even if it is assumed that such a highly precise and accurate routine clinical assay is possible, there remain two major unresolved fundamental problems with Herbert’s hypothesis. Firstly, as raised by several authors, there is uncertainty about what HoloTC actually represents. Does HoloTC concentration indicate recent absorption of vitamin B12, long-term body store or both? As stated by Chen: “The concept that a test can be used to diagnose both deficiency and malabsorption is problematic because the 2 defects are not identical. If holo-TC II truly reflects both processes, holo-TC II changes would perforce lose all diagnostic specificity for either process”. For the HoloTC immunoassay to have any clinical value in detecting the earliest onset of B12 deficiency, as Herbert intended, it would need to be either sensitive only to long-term status or be performed after a well-defined period of fasting.
Secondly, the model demands the reliable detection of a change in HoloTC concentration far smaller than the reported range of cut-off values. As noted by Sobczyńska-Malefora, “there has been little consensus with regard to the assigned cut-off to discriminate between replete and deficient states”. Herbert’s required change in HoloTC concentration from >37 to <30 pmol/L, to detect the earliest onset of B12 deficiency, is inconsistent with the reported range of cut-off values from 20 to 50 pmol/L. Furthermore, the use of various grey zones, for HoloTC concentration, is inconsistent with Herbert’s hypothesis in which Early Negative B12 Balance is detected by a universally agreed and closely specified fall in HoloTC concentration.
Questioning of Herbert’s hypothesis that HoloTC is the most sensitive marker of Early Negative Vitamin B 12 Balance, or his model for the sequential stages in the development of vitamin B12 deficiency, is likely to raise strong objections.Firstly, any model that is based on changes from individual normal analyte concentrations, instead of comparison with a universally accepted population normal, conflicts with the dogma that there must be a gold–standard test for vitamin B12 deficiency; either HoloTC or one a yet to be discovered.
Secondly, any model that requires a comparison of current analyte concentrations with a patient’s own normal levels would require proactive testing of all persons at risk, before the onset of any symptoms, to obtain their individual healthy baseline concentrations. Herbert himself suggested proactive testing, saying “serum holoTCIl should be measured every 5 y starting at age 55 because gradual loss of the ability to absorb vitamin B-12 occurs in everyone in a genetically determined, age-dependent pattern.”
A potential alternative to obtaining a longitudinal record for individuals at risk is the use of an algorithm to combine the results of a one-off test of all four analytes into a single parameter. A mathematical model for this has been devised and described in detail by Fedosov, and further tested and refined. This author is cautious about this approach because, as with all other current methods, it might apply well to a population but not necessarily to an individual. This would need extensive testing on a wide range of subjects, for various causes of vitamin B12 deficiency, before it could be validated.
Axis-Shield and others, in promoting the commercialisation of the HoloTC immunoassay, have relied on Herbert’s erroneous hypothesis that “Ho1oTCII falls below the bottom of its normal range long before total serum vitamin B-12 … falls below the bottom of its normal range”, and his flawed model for the staged development of vitamin B12 deficiency.
Herbert’s model is flawed because it assumes that a normal minimum concentration for HoloTC may be universally defined. As with total vitamin B12, evidenced by the failure to find such a single cut-off value for HoloTC and instead the discovery of a wide grey zone for HoloTC, the range of normal for an individual is much smaller than for the population. As stated by Herbert: “What is normal for one is not normal for another”.
Herbert’s hypothesis, that HoloTC will respond early to vitamin B12 depletion, before the onset of a clinical deficiency, is erroneous because it does not take into account how enterohepatic recycling regulates the HoloTC concentration. Where the cause of vitamin B12 deficiency does not disrupt the enterohepatic cycle, HoloTC will not be an early responder to a deficiency; there will be a Depletion period, in which total vitamin B12 falls, but HoloTC and the metabolites will only respond after the liver store is exhausted. When the cause of vitamin B12 deficiency does disrupt the enterohepatic cycle, the HoloTC will be an early responder but there will be no Depletion period; the metabolites will respond quickly when HoloTC falls below normal for the individual.
The HoloTC immunoassay cannot be used to measure vitamin B12 status any more reliably than total vitamin B12, or to predict the onset of a metabolic deficiency, because it is based on an erroneous hypothesis and a flawed model for the staged development of vitamin B12 deficiency.
Holotranscobalamin (HoloTC, Active-B12) and Herbert’s model for the development of vitamin B12deficiency: a review and alternative hypothesis
In Conclusion
To sum up, proponents believe the active-B12 test (or, as we prefer – the holoTC test) represents a major step-up over the regular blood test, because it gives a better picture of B12 that has any hopes of being absorbed. According to them, the test can make the task of B12 deficiency diagnosis easier for both the doctor and the patient.
However, others believe functional tests like homocysteine, and especially MMA, can discover functional B12 deficiencies more reliably. But even if MMA is better, measuring serum holotranscobalamin concentration could at least help verify results in cases of impaired renal function. We like this dual-biomarker approach.
Thank you for reading.